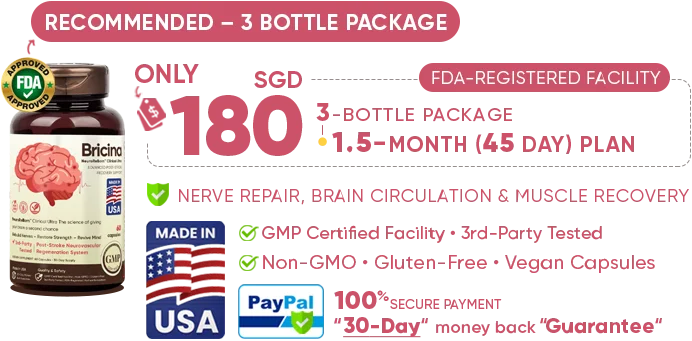
NeuroReBorn™ Clinical Ultra is manufactured in the United States at an
FDA-registered, GMP-certified facility. For a post-stroke recovery formula, this ensures safety, purity, and consistent clinical-grade results.U.S. Quality Standards & Strict Process Control
- FDA-registered facility: regulated production, documentation, and quality control.
- cGMP certified: strict guidelines for cleanliness, precision, and manufacturing accuracy.
- Independent batch testing: potency, purity, and safety verified before release.
High-Purity, Clinically Researched Ingredients
All active ingredients are sourced from trusted non-GMO suppliers and verified by Certificate of Analysis (COA) for identity, purity, and strength.
- Non-GMO raw materials with clean extraction processes.
- Standardized extracts for consistent neurological support.
Advanced Capsule Technology
- Vegetable HPMC capsules: gentle and safe for long-term daily use.
- Controlled disintegration: optimized absorption for seniors.
Transparency, Safety & Patient Confidence
Every batch of NeuroReBorn™ Clinical Ultra is traceable from raw materials to finished product.
- COA and batch numbers ensure full traceability.
- No banned or hidden substances—fully compliant with U.S. regulations.
Why “Made in USA” Matters for Stroke Recovery
- Consistent results thanks to strict U.S. quality systems.
- Higher trust for caregivers and senior patients.
- Internationally respected origin with strong scientific oversight.
NeuroReBorn™ Clinical Ultra – Made in USA represents a commitment to safety, science, and dependable neurological recovery for stroke survivors.